

Lonza TheraPEAK™ MSCGM™ Mesenchymal Stem Cell Growth Medium BulletKit™
TheraPEAKTM MSCGMTM Mesenchymal Stem Cell Growth Medium BulletKitTM including the basal medium and supplements, which is GMP-produced and serum-free, 1 L.. Catalog #: BEBP18-936
Product Overview
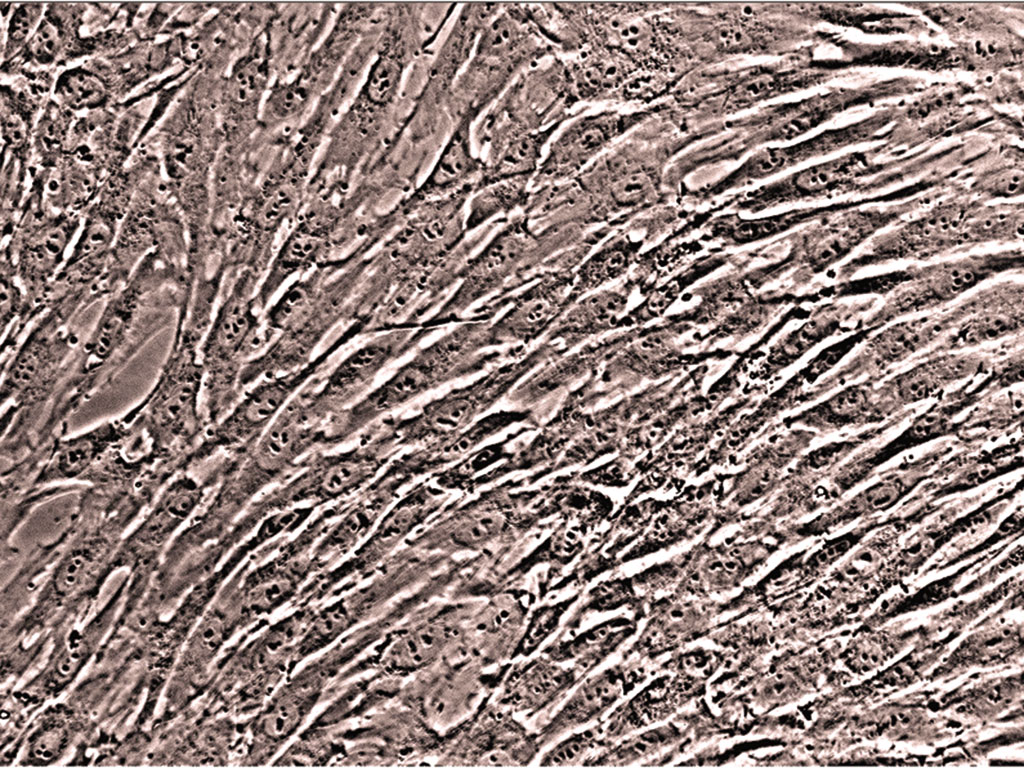
Stem cells possess the unique ability to proliferate as an undifferentiated cell type, then, when properly stimulated, differentiate into multiple lineages of cells. Lonza’s TheraPEAK™ MSCGM™ Mesenchymal Stem Cell Growth Medium is a serum-free medium designed to proliferate human bone marrow derived mesenchymal cells in an undifferentiated state. The medium has been specifically designed and tested to ensure that the medium neither promotes spontaneous differentiation of cells nor inhibits cell differentiation to osteogenic, chondrogenic, or adipogenic lineages (when properly stimulated with an appropriate differentiation media). Serum-free media, such as TheraPEAK™ MSCGM™ Mesenchymal Stem Cell Growth Medium, are intended to support cell growth without serum supplementation. Lonza's TheraPEAK™ MSCGM™ Mesenchymal Stem Cell Growth Medium is of primary interest to those seeking maximal control over research and manufacturing variables. TheraPEAK™ MSCGM™ Mesenchymal Stem Cell Growth Medium does not contain phenol red or antibiotics.
Partial List of Cells Proliferated with TheraPEAK™ MSCGM™ Mesenchymal Stem Cell Growth Medium*:
Human bone marrow derived stem cells
Human periodontal ligament derived stem cells
Human adipose derived stem cells
TheraPEAK™ MSCGM™ Mesenchymal Stem Cell Growth Medium is offered as a BulletKit™ Medium (catalog no. BEBP18-936) which includes both the basal media and the necessary supplements for proliferation of human bone marrow derived mesenchymal stem cells.
*List of cells generated from customer data. Lonza only routinely uses this medium with Lonza’s human bone marrow derived mesenchymal stem cells and can therefore only guarantee the successful use of this media with these cells. All TheraPEAK™ Media are GMP products intended to support further manufacturing and are produced to GMP raw material standards. These media products conform with cGMP manufacturing compliance expectations for medical devices including 21 CFR Part 820 and ISO 13485. TheraPEAK™ Media products are produced at FDA registered manufacturing sites with an ISO 13485 certified quality management system. All research use only formulations are produced according to ISO:9001.
Benefits
Serum-free and regulatory friendly
No need for attachment matrix to plate cells
Optimized for multiple expansion of all types of hMSCs
Supports multi-lineage differentiation
Provided in BulletKit™ format, includes basal medium and supplement
Custom packaging available to meet scale up needs
Can be used in small or large scale therapeutic applications
Catalog #: BEBP18-936
Please email us at cs@medikonia.com for any enquiry. To place an order, please include the catalog number(s) of the product(s) in the email.