
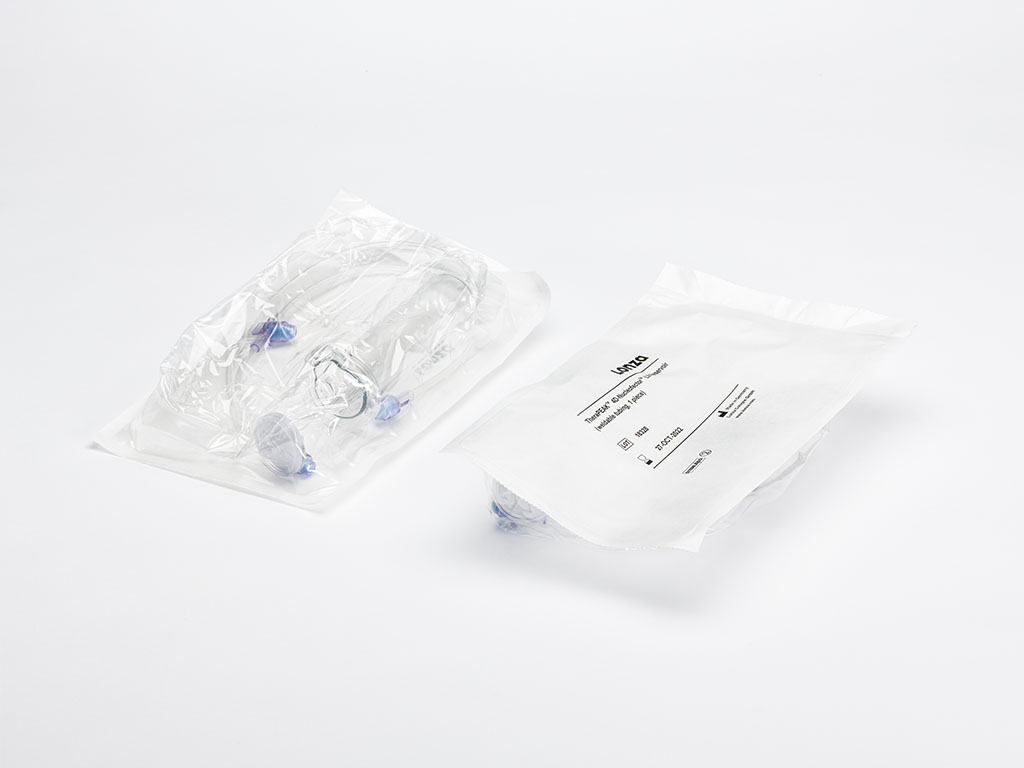


Lonza TheraPEAK® 4D-Nucleofector® LV Reservoirs, weldable (5 pcs)
For use in combination with TheraPEAK® Nucleocuvette® Cartridges, supporting the use of the 4D-Nucleofector® LV Unit in a GMP environment. Catalog #: G4LR-1501W
Product Overview
TheraPEAK® Nucleofector® Consumables are aimed to support the use of the 4D-Nucleofector® LV Unit as manufacturing equipment in a GMP manufacturing process for advanced therapeutic medicinal products (ATMPs). In such a process, the 4D-Nucleofector® LV Unit and its consumables may be used for non-viral, electroporation-based genetic modification of primary cells, for example T cells or hematopoietic stem cells.
Like their research-grade counterparts, TheraPEAK® 4D-Nucleofector® LV Reservoirs are the Lonza designed vessels for transferring the cell suspension sample into a TheraPEAK® LV Nucleocuvette® Cartridges and to collect the sample after transfection.
In contrast to the research-grade LV Reservoir, the TheraPEAK® LV Reservoirs come with a new design:
Injection-molded body and cap glued together to provide a fully closed environment
Lower PVC tubing allows for sterile welding to a TheraPEAK® LV Nucleocuvette® Cartridge (input line) or downstream equipment (output line)
Upper PVC tubing enables filling from top either from upstream equipment (sample input) or TheraPEAK® LV Nucleocuvette® Cartridge (transfer of transfected sample into collection reservoir)
Sterile welding:
The PVC tubing allows for sterile welding to a TheraPEAK® LV Nucleocuvette® Cartridge or upstream/downstream equipment. Alternatively, sterile connections can be established manually via Luer connectors.
TheraPEAK® 4D-Nucleofector® LV Reservoirs are tested for
• Endotoxin (Ph. Eur. 2.6.14)
• Cytotoxicity (EN ISO 10993-5:2009)
• Visible particles (Ph. Eur. 2.9.20)
• Sub-visible particles (Ph. Eur. 2.9.19)
• Biology (Nucleofection® Experiment)
Disclaimer: TheraPEAK® Nucleofector® Products are produced according to applicable GMP standards and are intended to support GMP manufacturing. TheraPEAK® Nucleofector® Cartridges and Reservoirs are produced at manufacturing sites with an ISO13485 certified quality management system. TheraPEAK® Nucleofector® Products are not intended for in vivo and/or diagnostic purposes
Benefits
Single-use consumables
Use of biocompatible material, medical grade (where available)
Produced at an ISO13485:2016 certified site in a class C environment
Sterilized by ethylene oxide (validated process)
Double-layer packaging supporting the clean room cascade
Validated manufacturing and testing methods
Applications
Supporting use of 4D-Nucleofector® LV Unit in GMP manufacturing
Content
5 TheraPEAK® 4D-Nucleofector® LV Reservoirs (weldable tubing)
Catalog #: G4LR-1501W
Please email us at cs@medikonia.com for any enquiry. To place an order, please include the catalog number(s) of the product(s) in the email.